Engineered 3D Breast Tumor Model
Tumor models are important tools in cancer research because they replicate certain aspects of native tumor environments. We have developed a 3D model of primary breast tumors that offers several key features: 1) it reproduces the architecture of native tumors in terms of relative distribution of cancer cells and stromal cells; 2) it allows tuning the biochemical composition of the extracellular matrix by including specific matrix proteins such as collagen, fibronectin, and hyaluronic acid; and 3) adjust mechanical properties of the extracellular matrix by changing the concentration of the structural proteins. We leverage this organotypic model to study several key questions: What are specific mechanisms by which stromal cells such as cancer-associated fibroblasts interact with breast cancer cells? How different signaling molecules from the extracellular matrix promote tumorigenic functions of breast cancer cells such as local invasion? What are predominant drivers of stemness and resistance of breast cancer cells to chemotherapy and targeted therapy? Answering these questions will help develop novel treatment strategies to more effectively treat breast cancers, especially the subtypes such as triple negative breast cancer that have very limited advanced therapy options. The images show (left) a confocal reconstruction of the organotypic tumor model show with breast cancer cell mass (blue) and cancer-associated fibroblasts (green) in a collagen matrix (not shown), and (right) breast cancer cell mass (green), cancer-associated fibroblasts (red), and collagen (cyan).

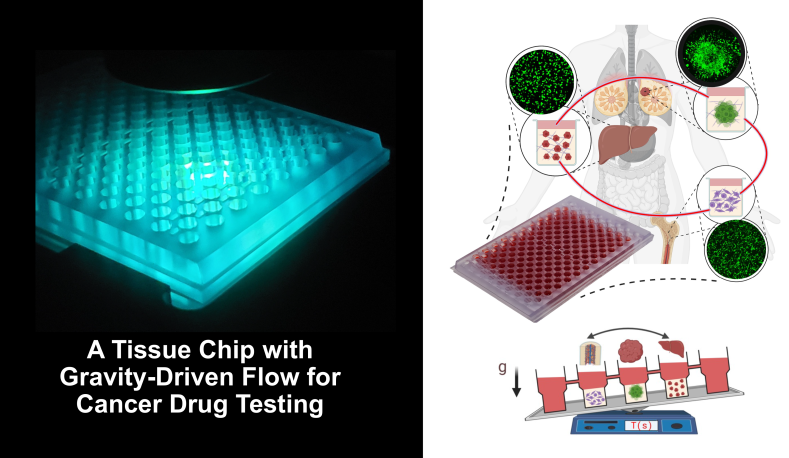
Organs-on-a-Chip for Cancer Treatments
Cancer treatments can cause significant, and at times, intolerable toxicity in normal tissues such as in bone marrow, gastrointestinal tract, and liver of cancer patients. This creates a key obstacle against treating of patients. There is currently a major need for novel approaches that can reliably predict efficacy of cancer treatments against tumor cells and their potential toxicities to normal tissues. We have developed an organs-on-a-chip technology to address this need. This device has tissue culture compartments for breast tumor, bone marrow, and liver. This design is adapted a plate format to allow testing many drugs simultaneously. The compartments are fluidically connected to resemble circulation, which is achieved with gravity-driven flow to eliminate peripherical flow control devices. By leveraging this technology, our goal is to use different FDA-approved drugs and their combinations and evaluate how effectively they eradicate cancer cells and to what extent they are toxic to normal tissues, determine specific mechanisms of efficacy and toxicity, and offer strategies to design more effective and less toxic treatment modalities. Our long-term goal is to use this device for personalized cancer therapy where different treatments tested against a patient’s own cells to guide clinical decision making.
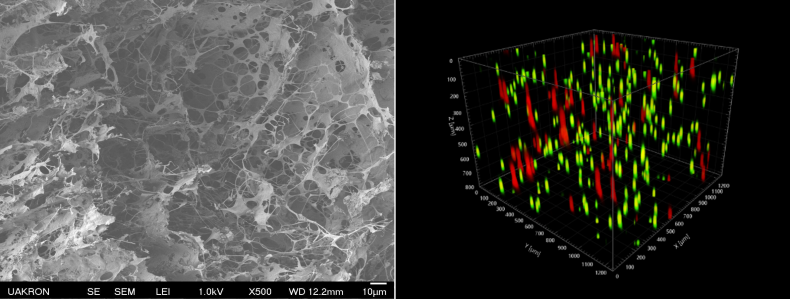
Tissue Engineered Model of Bone Marrow Metastasis of Breast Cancer
Estrogen receptor-positive breast cancer (ER+ BC) comprises about 75% of all breast cancers. Despite the success of targeted endocrine therapies, recurrence of ER+ BC in metastatic sites is common. Even after 5 years of adjuvant endocrine therapy, up to 52% of patients remain at risk of distant recurrence and death within 20 years after diagnosis. Survival of cancer cells in bone marrow to form metastases accounts for about 70% of advanced ER+ BC disease. We have developed a 3D culture model of ER+ BC and mesenchymal stromal cells (MSCs) in bone marrow to determine major mechanisms of survival and resistance of ER+ BC cells to endocrine therapies. The images show (left) an engineered3D extracellular matrix and (right) a confocal reconstruction of the model with ER+ BC shown in red and MSCs in green suspended in the extracellular matrix.
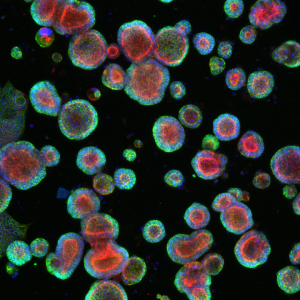
Tissue Engineered Patient-Specific Colorectal Tumor Organoids
Organoids represent a tissue model for basic and translational research. We have developed a technology that enables maintaining patient-derived colorectal cancer cells in culture to develop tumor organoids. The organoids mimic certain structural properties and biological functions of colon tissue. Our objective is to leverage this model and identify key interaction mechanisms between stromal cells such as cancer-associated fibroblasts and tumor organoids that promote a stem cell-like phenotype and render cancer cells drug resistant. The image shows patient-derived colorectal tumor organoids. Colors represent different biomarkers: Actin, a protein in the cytoskeleton that help cells move and keep their shape (red); Beta-catenin, a protein showing polarization of the organoids, much like in the colon tissue (green); Nuclei of cells, where the genetic material (DNA) is stored (blue).