Engineering tumor microenvironment for drug discovery
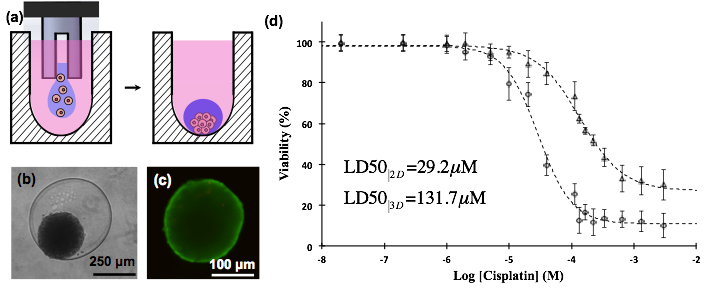
Discovery of effective therapeutics for treatment of solid tumors requires screening of candidate compounds with physiologic tumor models. We have developed a new technology that uses the immiscibility of two aqueous phases to robotically micropattern 3D tumor spheroids from cancer cells in high throughput microwell plate formats (Fig.1a). Resulting spheroids show normal morphology (Fig.1b), are fully viable (Fig.1c), and allow high throughput drugs testing with a tumor model (Fig.1d). Spheroids express key biological markers of tumors in vivo. This technology is currently used for compound screening to identify novel chemical probes with chemotherapeutic benefits. In addition, the effect of different components of tumor microenvironment on the response of cancer cells to therapeutics is under investigation.
Fig.1 (a) An aqueous two-phase system is used to confine cancer cells within a nanoliter drop and facilitate aggregation of cells into a spheroid, (b) spheroid of MDA-MB-157 breast cancer cell formed within the aqueous drop, (c) spheroid is fully viable (green color represents live cells and red color represents dead cells), (d) spheroids of A431 skin cancer cells (triangles) exhibit greater resistance to a clinical chemotherapy drug (cisplatin) compared to the 2D culture of cancer cells.
References:
S.L. Ham, P.S. Thakuri, M. Plaster, J. Li, K.E. Luker, G.D. Luker, H. Tavana, “Three-dimensional tumor model mimics stromal – breast cancer cells signaling” Oncotarget 9 (2018) 249-267.
S.L. Ham, R. Joshi, G.D. Luker, H. Tavana, “Engineered breast cancer cell spheroids display biologic properties of solid tumors” Adv. Healthcare Mater. 5 (2016) 2788-2798.
P.S. Thakuri, S.L. Ham, G.D. Luker, H. Tavana, “Multi-parametric analysis of oncology drug screening with aqueous two-phase tumor spheroids” Mol. Pharm. 13 (2016) 3724-3735.
S.L. Ham, E. Atefi, D. Fyffe, H. Tavana, “Robotic production of cancer cell spheroids with an aqueous two-phase system for drug testing”, J. Vis. Exp. 98 (2015) e52754
S. Lemmo, E. Atefi, G.D. Luker, H. Tavana, “Optimization of aqueous biphasic tumor spheroid microtechnology for anti-cancer drug testing in 3D culture” Cell. Mol. Bioeng. 7 (2014) 344-354.
E. Atefi, S. Lemmo, D. Fyffe, G.D. Luker, H. Tavana, “High throughput, polymeric aqueous two-phase printing of tumor spheroids” Adv. Func. Mater. 24 (2014) 6509-6915.
Neural tissue engineering using embryonic stem cells
Neural tissues have limited capacity to regenerate ravaged and lost cells. Therefore, the use of therapeutic interventions such as cell replacement therapies is crucial for treatment of neurodegenerative disorders. We have developed a micro-engineered heterocellular culture system that resembles embryonic development in terms of direct intercellular interactions and induces neural differentiation of embryonic stem cells. A polymeric aqueous two-phase system (ATPS)-mediated microprinting allows precise localization of stem cells over a layer of supporting stromal cells to form a defined stem cell niche (Fig.2). Stem cells receive physical and chemical cues from the stromal cells and differentiate toward specific neural lineages such as neurons, astrocytes, and oligodendrocytes. Cellular and molecular modulation of the differentiation process is under investigation.
Fig.2 (a) Aqueous two-phase cell printing on cells, (b) cell-cell contact mediated neuronal differentiation of embryonic stem cells printed on stromal cells (red fluorescent represents neuron specific tubulin TuJ), (c) higher magnification image of neuronal processes.
References:
R. Joshi, J.B. Buchanan, P.S. Thakuri, J. Li, H. Tavana, “Microprinted embryonic stem cell niches reveal compounding effect of colony size on stromal cells-mediated neural differentiation” Adv. Healthcare Mater. 7 (2018) 1700832.
R. Joshi, J.B. Buchanan, H. Tavana, “Self-regulatory soluble factors of embryonic stem cells in co-culture with stromal cells enhance neural differentiation” Integr. Biol. 9 (2017) 418-426.
R. Joshi, J. Buchanan, S. Paruchuri, N. Morris, H. Tavana “Molecular analysis of stromal cells-induced neural differentiation of mouse embryonic stem cells” PLoS One 11 (2016) e0166316.
R. Joshi, J. Buchanan, H. Tavana, “Colony size effect on neural differentiation of embryonic stem cells microprinted on stromal cells” Conf. Proc. IEEE Eng. Med. Biol. Soc. (2016) 4173-4176.
H. Tavana, B. Mosadegh, P. Zamankhan, J.B. Grotberg, S. Takayama, “Microprinted feeder cells guide embryonic stem cell fate”, Biotech. Bioeng., 108 (2011) 2509-2516.
H. Tavana, B. Mosadegh, S. Takayama, “Polymeric aqueous biphasic systems for non-contact cell printing on cells: Engineering heterocellular embryonic stem cell niches”, Adv. Mater., 22 (2010) 2628-2631.
High-throughput screening of cancer cell migration and invasion
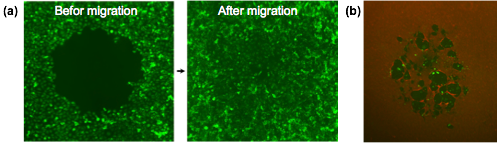
Migration and invasion of cancer cells are key processes for the progression of malignant tumors. Understanding mechanisms that regulate these events will help design new strategies to combat cancer. We have developed a novel microtechnological assay that enables studying migration and invasion of cancer cells in vitro. We pattern cells in microwell plates as a monolayer containing a circular gap region. This gap serves as migration “niche” for surrounding cells such that cells move into the gap region and occupy it over time (Fig.3a). The rate of closure of the gap by cancer cells correlates with their motility and aggressiveness. We have also developed a platform to study invasion of motile cancer cells through extracellular matrices (Fig.3b). These technological platforms are currently being utilized to screen candidate compounds for “hits” that can prevent cancer cell motility.
Fig.3 (a) Migration of MDA-MB-231 breast cancer cells and (b) invasion of caner cells (green fluorescent) through extracellular matrix (red fluorescent type I collagen).
References:
S.L. Ham, S. Nasrollahi, K.N. Shah, A. Soltisz, S. Paruchuri, Y.H. Yun, G.D. Luker, A. Bishayee, H. Tavana, “Phytochemicals potently inhibit migration of metastatic breast cancer cells” Integ. Biol. 7 (2015) 792-800.
S.Lemmo, S. Nasrollahi, H. Tavana, “Aqueous biphasic cancer cell migration assay enables robust, high throughput screening of anti-cancer compounds”, Biotech. J., 9 (2014) 426-434.
H. Tavana, K. Kaylan, T. Bersano-Begey, K.E. Luker, G.D. Luker, S. Takayama, “Rehydration of polymeric, aqueous, biphasic system facilitates high throughput cell exclusion patterning for cell migration studies”, Adv. Func. Mater., 21 (2011) 2920-2926.
H. Tavana, A. Jovic, B. Mosadegh, Q.Y. Lee, X. Liu, K.E. Luker, G.D. Luker, S.J. Weiss, S. Takayama, “Nanoliter liquid patterning in aqueous environments for spatially-defined reagent delivery to mammalian cells”, Nature Mater., 8 (2009) 736-741.
Pulmonary biofluid mechanics
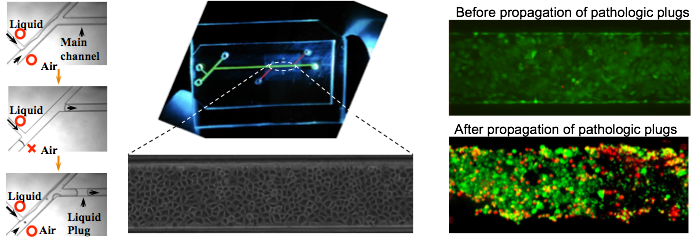
Lung airways are normally lined with a thin liquid film secreted and maintained by airway epithelial cells. Pulmonary surfactant within the liquid film reduces the surface tension at the air-liquid interface of airways to maintain non-cartilaginous distal airways open at low lung volumes. Abnormalities in the production or surface activity of lung surfactant in various respiratory disorders result in the instability of the air-liquid interface of distal airways and formation of a liquid plug that obstructs airflow to the lung periphery and impedes the gas exchange process. In addition, these pathologic liquid plugs exert abnormal mechanical stresses on the airway epithelium. We are studying this phenomenon using a novel microfluidic model of lung small airways. The device accommodates generation of well-defined liquid plugs (Fig.4a), on-chip culture of lung epithelial cells (Fig.4b), and investigation of the effect of pathologic fluid flow on airway epithelium (Fig.4c).
Fig.4 (a) on-chip liquid plug generation, (b) confluent monolayer of lung airway epithelial cells, (c) cell damage due to flow of pathologic liquid plugs (green fluorescent represents live cells and red fluorescent represents dead cells).
References:
H. Tavana, P. Zamankhan, P.J. Christensen, J.B. Grotberg, S. Takayama, “Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model”, Biomed. Microdev., 13 (2011) 731-742.
H. Tavana, C.-H. Kuo, Q.Y. Lee, B. Mosadegh, D. Huh, P.J. Christensen, J.B. Grotberg, S. Takayama, “Dynamics of liquid plugs of buffer and surfactant solutions in a micro-engineered pulmonary airway model”, Langmuir, 26 (2010) 3744-3752.
H. Tavana, D. Huh, J.B. Grotberg, S. Takayama, “Microfluidics, lung surfactant, and respiratory disorders”, Lab. Medicine, 40 (2009) 203-209.